Abstract
Acute graft versus host disease (aGVHD) is a leading cause of non-relapse mortality after allogeneic peripheral blood hematopoietic cell transplant (PB-HCT). JAK inhibition has been shown to be an effective treatment for steroid refractory aGVHD. We performed a single arm, open label, phase I trial to evaluate the safety of a best-in-class balanced JAK1/2 inhibitor, baricitinib (Bari), for the prevention of aGVHD after HLA-matched, allogeneic PB-HCT (Choi et al. Leukemia2018; Kim et al. Leukemia, 2022).
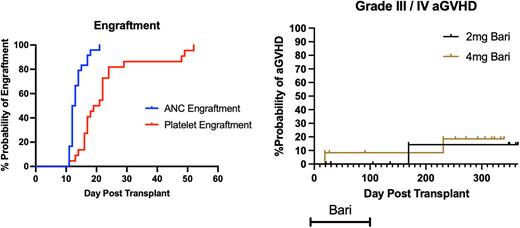
The study enrolled 24 subjects into two dosing cohorts (2mg and 4mg daily) from 2019 - 2022. The data cut-off for this analysis was June 1, 2022. The primary objectives were to determine the incidence of graft failure at 28 days post HCT and the cumulative incidence of grade III-IV acute GVHD by day 100. The secondary objective was to determine day 180 treatment related mortality. Subjects were treated with Bari 2mg or 4mg (n = 12/cohort) oral daily from days -3 to +100, followed by a taper. All subjects received standard of care GVHD prophylaxis with tacrolimus and mini-methotrexate +/- thymoglobulin (n=19) or post-transplant cyclophosphamide (pTCy) plus mycophenolate mofetil and tacrolimus (n=5).
At data cut, the median follow up was 320 days (63-368 days). The median age was 57 (range 28-72), and diagnoses included AML (16), MDS (6), and ALL (2). All subjects with AML were required to be in CR/CRi and 8 were MRD positive by flow cytometry at the time of transplant. Ten recipients had PB-HCT from sibling donors and 14 had PB-HCT matched unrelated donors. Conditioning included myeloablative regimens (MAC, n=19) or reduced intensity (RIC, n=5). GVHD prophylaxis included tacrolimus /methotrexate for all subjects in the 2mg cohort and pTCy was used in 5 / 12 subjects in 4mg dosing cohort. Thymoglobulin was allowed for MUD recipients (n=5). No events of primary graft failure were observed. The median ANC engraftment was 12.5 days (range 12-21). The median platelet engraftment was 20 days (range 13-52). Treatment related mortality at day 180 was 8% (2/24). The cumulative incidence of grade III/IV aGVHD at day 100 was 4% (1/24, occurring in the 4mg cohort), while the overall incidence of grade III/IV aGVHD at any time was 12.5% (3/24) and two of these cases occurred after Bari discontinuation. The one case of grade III aGVHD prior to day 100 was a steroid-responsive lower GI GVHD that occurred at day 21 in the 4mg cohort and did not recur with taper of immune suppression and Bari. Grade II - IV aGVHD occurred in 33% (8/24, 4 in the 2mg cohort and 4 in the 4mg cohort). Two cases were steroid refractory (one in 2mg and one in 4mg cohorts). All cases of grade II aGVHD were responsive to steroids. We found no association between GVHD prophylaxis backbone regimen and incidence of aGVHD. 11 cases of chronic GVHD (3 mild and 8 moderate severity) have been reported. Of the moderate cGVHD cases, 4 developed in the 2mg cohort and 4 in the 4mg cohort. No unexpected grade 3/4 adverse events (AE) were observed. Thromboembolic disease was an AE of special interest. Four DVTs were observed: 1 in the 2mg cohort vs. 3 in 4mg cohort. All subjects in the 4mg cohort had pulmonary embolism occurring while off Bari, one occurring at 10 months post-transplant, one at day 141 and another at day 152. Of the evaluable cases at day 100, 14/15 (93%) receiving MAC vs. 4/5 (80%) receiving RIC conditioning achieved >95% bone marrow donor engraftment, whereas, 11/16 (69%) receiving MAC vs. 4/4 (100%) receiving RIC had mixed peripheral blood CD3 T-cell chimerism. Of those with mixed CD3 chimerism at day 100, 12 remain in remission and 3 have relapsed. The incidence of disease relapse at 1 year was 21%, with 2 relapses occurring in the 2mg cohort and 3 in the 4mg cohort. Of the 8 cases with MRD positivity at transplant, 2 relapsed within 3 months of transplant and both had AML. The 1-year overall survival was 70%. The one-year refined GVHD relapse free survival (GRFS) was 33%.
In conclusion, Baricitinib is safe after HLA-matched, allogeneic PB-HCT with no episodes of primary graft failure and low rates of grade III/IV acute GVHD while on therapy. A follow up efficacy study to evaluate extended duration of the 2mg dose of Bari focused on AML patients receiving HLA matched transplants and myeloablative conditioning is planned.
Disclosures
Schroeder:Fortis: Research Funding; Cellect Inc: Research Funding; Genentech Inc: Research Funding; Incyte: Research Funding; Seagen Inc.: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Fehniger:Wugen: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties; Affimed: Other: Scientific Advisory Board, Research Funding; Indapta: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board; Orca Bio: Current holder of stock options in a privately-held company; HCW Biologics: Research Funding; ImmunityBio: Research Funding; BMS: Other: Provision of drug for investigator initiated trial. Ghobadi:Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding; Wugen Inc: Consultancy; Atara: Consultancy; Celgene: Consultancy. Vij:CareDx: Honoraria; Janssen: Honoraria; Biegene: Honoraria; Legend: Honoraria; BMS: Honoraria, Research Funding; Pfizer: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Adaptive: Honoraria; Harpoon: Consultancy; Oncopeptides: Honoraria; Takeda: Honoraria, Research Funding. DiPersio:RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
baricitinib for GVHD prophylaxis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal